Subscribe to Our Newsletters
Feedstuffs is the news source for animal agriculture
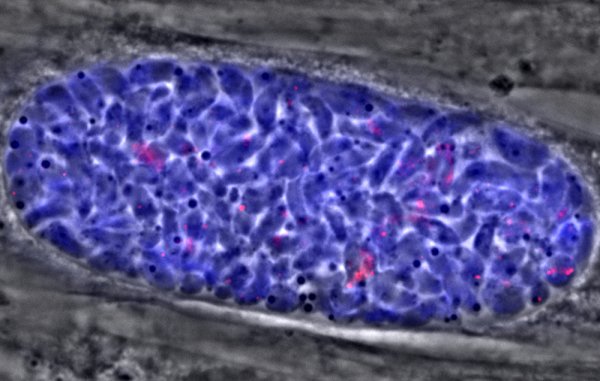
Parasite contracted from consuming undercooked, contaminated meat or coming in contact with cat feces containing oocyst form of toxoplasma.
July 12, 2017
You May Also Like