How is antibiotic use changing?
April 24, 2015

I think we can all agree it’s in everyone’s best interest to have access to effective antibiotics for people and for animals. It’s critical for public health — and vital for livestock production and well-being.
That’s why it’s also important for those of us in animal agriculture to demonstrate we’re doing our part to protect antibiotic effectiveness and in doing so, help maintain confidence in food safety. In pursuit of those objectives, labels of certain classes of antibiotics — those used in both animals and people — will be transitioning. In 2012, the U.S. Food and Drug Administration (FDA) put forth plans to change the way feedand water-based antimicrobials are used in cattle and other production animals. Three documents provide the details: Guidance for Industry No. 209, Guidance for Industry No. 213 and Veterinary Feed Directive (VFD) 21 CFR 558. The first one (No. 209) is the “what” component. It establishes the key principles: The use of medically important antimicrobial drugs in food-producing animals should be limited to:
• Uses necessary for assuring animal health
• Uses that include veterinary oversight
The second report (No. 213) is the “how” component. It provides a road map for implementing those two principles by addressing issues such as product labeling.
The third report (CFR 558) aims to modernize the VFD process. It’s an effort to streamline procedures while providing greater oversight.
The FDA is taking these steps to protect public health. On that front, it’s the right policy for the right reasons. The FDA calls antimicrobial resistance “a mounting public health problem of global significance.” The FDA fears that if certain antimicrobials are overused in animals, they’ll become less effective in humans.
When the FDA released these reports, the agency said it was pursuing a “voluntary approach” for compliance. The FDA plans to evaluate progress three years after final publication and “consider further actions as warranted.” Guidance for Industry No. 213 was finalized in December 2013. Now the clock is ticking.
From an industry perspective this might not seem ideal. However, it’s preferable to many alternatives, such as elimination of medications or Congressional oversight. No one wants those, either. These steps also go a long way toward protecting long-term access to antibiotics. The voluntary approach was wise for all parties. It gives everyone time to understand the policies, figure out how to comply with them and determine the most efficient process for transitions.
The industry is not going to lose all feed-grade antibiotics. But the way certain products are used will change. In modern livestock production, antibiotics are used in four ways:
1. Treat animals diagnosed with an illness
2. Control the spread of an illness in a herd
3. Prevent illness in healthy animals
4. Enhance growth or improve feed efficiency
The FDA says that “medically important” antibiotics would still be available for those first three, therapy-related uses under the supervision of a veterinarian. But these products could not be used for the fourth option: enhancing growth or improving feed efficiency (see table).
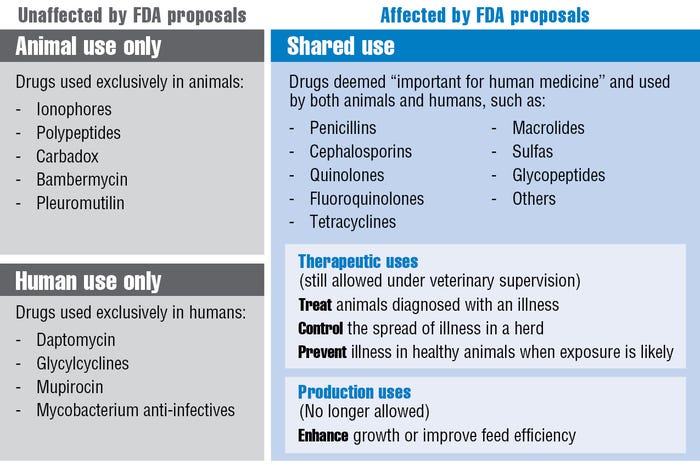
(“Medically important” refers to drugs considered important for therapeutic use in humans.)
Note that prevention remains an appropriate therapeutic use of antibiotics as determined by a veterinarian. It’s also important to clarify that performance claims still can be made for drugs that are used exclusively in animals.
Like many animal health companies, Elanco has publicly announced its intention to fully comply with the FDA guidance. It’s Elanco’s responsibility to help ensure that antimicrobials are used responsibly to protect the health of animals and humans as well as the safety of the food supply.
Moving forward, Elanco will make investments in innovative alternatives that could lessen the reliance on antibiotics while preserving the efficacy of antibiotics for humans, animals and food safety. When antibiotics are used, we want to ensure there is appropriate veterinary oversight and that they are used responsibly.
Courtesy of Elanco Animal Health
USBBUNON01097
You May Also Like



