- Agribusiness News
- Nutrition & Health
- Swine Health and Nutrition
- Poultry Health and Nutrition
- Beef Health and Nutrition
- Dairy Health and Nutrition
Domestic sales of antimicrobials for food-producing animals decline
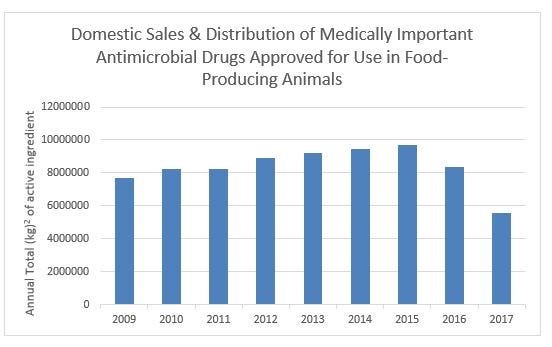
Domestic sales and distribution of all medically important antimicrobials decreased 41% since 2015 and 28% since first year of reported sales in 2009.
The U.S. Food & Drug Administration announced Dec. 18 that domestic sales and distribution of all medically important antimicrobials intended for use in food-producing animals decreased 33% between 2016 and 2017.
FDA said its “2017 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals” also shows that domestic sales and distribution of all medically important antimicrobials decreased 41% since 2015 (peak year of sales/distribution) and decreased 28% since the first year of reported sales in 2009.
While sales data do not necessarily reflect actual antimicrobial use, the reduction in sales volume observed in 2016 and 2017 is an important indicator that ongoing efforts to support antimicrobial stewardship are having a significant impact, FDA said.
The 2017 summary report is the first report to include data submitted after the full implementation of Guidance for Industry (GFI) #213. Based on recommendations in GFI #213, all production uses (e.g., growth promotion and feed efficiency) of medically important antimicrobials in the feed or drinking water of food-producing animals were eliminated, and such drugs can now be used only for therapeutic purposes under veterinary oversight (such as under veterinary feed directives [VFDs]).
“Optimizing how medically important antimicrobial drugs are used and limiting their use to only when necessary to treat, control or prevent disease will help to preserve the effectiveness of these drugs for fighting disease in both humans and animals. While it’s impossible to completely outrace antimicrobial resistance, we can take important steps now to slow its pace and reduce its impact on both human and animal health,” FDA commissioner Scott Gottlieb said.
FDA said it is critical to remember that the agency’s primary goal with initiatives like GFI #213 and the Center for Veterinary Medicine’s (CVM) five-year action plan is not to reduce antimicrobial sales volume butm rather, to support the implementation of good antimicrobial stewardship practices in order to slow the development of antimicrobial resistance.
Although sales data provide insight regarding antimicrobial drugs entering the marketplace, FDA said it is also important to consider additional sources of information when assessing the progress of efforts to foster judicious antimicrobial use, including actual usage data, animal demographics, animal health data and resistance data. The agency continues to work with federal, academic and industry partners to obtain more information about how, when and why animal producers and veterinarians use medically important antimicrobial drugs in food-producing animals.
FDA said it plans to publish a report in 2019 that integrates and analyzes these other data sources to more fully assess the progress of antimicrobial stewardship efforts.
When analyzing the report, FDA said readers should consider:
Sales and distribution information does not represent actual use of the products. It is important to acknowledge that these data are sponsors’ estimates of product sales and are not intended to be a substitute for actual usage data. For example, veterinarians and animal producers may purchase drugs but never actually administer them to animals, or they may administer the drugs in later years.
Before making a direct comparison between the quantity of antimicrobial drugs sold for use in humans and for use in animals, readers should consider:
1. There are many more animals than humans. For instance, there are approximately 320 million people in the U.S., while U.S. Department of Agriculture records indicate that about 9 billion chickens are slaughtered annually.
2. There are differences in physiology and weight between humans and animals; the average adult human weighs 182 lb., while a beef steer weighs about 1,363 lb.
3. Different animal species metabolize drugs differently, meaning that some may require more of the drug to be effective or may need to be treated for a longer period of time.
“The successful implementation of changes, such as those outlined in GFI #213, depend heavily on the commitment of our key partners and stakeholders, including animal pharmaceutical and feed industries, the animal agriculture community, the veterinary community and other federal and state agencies, such as the U.S. Department of Agriculture,” Gottlieb said. “At FDA, we continue to renew our commitment to work in consultation with all of our stakeholders and across government to identify ways to develop and disseminate information on antimicrobial stewardship. This ranges from collaborating with consumer groups to providing information to animal producer groups and veterinarians on good stewardship practices to working with veterinary medical associations and academic institutions to create veterinary curricula with the most up-to-date information on antimicrobial stewardship.”
While the 2017 annual summary report demonstrates measurable progress and FDA appreciates all the efforts by stakeholders, more work is needed to address antimicrobial resistance. CVM’s five-year action plan outlines additional steps the agency intends to take to foster antimicrobial stewardship in veterinary settings.
About the Author(s)
You May Also Like



