thumbnail
Agribusiness News
Agriculture emissions fall to lowest levels in 10 yearsAgriculture emissions fall to lowest levels in 10 years
Farmers and ranchers across America are at the forefront of reducing greenhouse gas emissions through voluntary conservation initiatives and incentives based on market principles.
Subscribe to Our Newsletters
Feedstuffs is the news source for animal agriculture

.png?width=700&auto=webp&quality=80&disable=upscale)

.png?width=700&auto=webp&quality=80&disable=upscale)

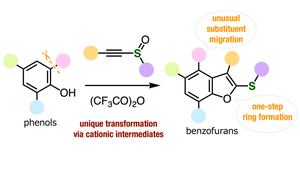

.png?width=300&auto=webp&quality=80&disable=upscale)



.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)









.png?width=300&auto=webp&quality=80&disable=upscale)








