If using mitigant with nutritionally important ingredients such as vitamins, assure that mitigant will not negatively affect ingredient's nutritional value.
June 11, 2019

Sponsored Content
By Mark Bienhoff and Jon Bergstrom*
*Dr. Mark Bienhoff is with Kemin Industries, and Dr. Jon Bergstrom is with DSM Nutritional Products.
Over the last decade, the landscape regarding potential pathogen contamination of feed in animal production has been evolving, from discussions focused on treatment to increasing considerations for risk mitigation.
Many forces are driving this change — consumer demands, producer efforts to reduce antibiotic usage, changes in the regulatory landscape and, of course, the risk of emerging animal diseases on a global level.
The porcine epidemic diarrhea virus (PEDV) outbreak that swept across the U.S. pork industry beginning in 2013 was a wake-up call for all stakeholders involved in agriculture, food and trade. The risk of feed contamination was previously an overlooked part of on-farm biosecurity programs. However, feed was quickly identified as a possible transmission route for PEDV.
Similarly, more recent studies demonstrated certain viruses, including some foreign animal diseases, remain viable in some common feed ingredients after simulated contamination under transboundary shipping conditions.
Recent research from Kansas State University has further explored the transmission of African swine fever virus through feed — demonstrating that it is a plausible infection vehicle, with risk positively correlating to the number of animal exposures (i.e., the more often an animal comes into contact with contaminated feed, the more likely they are to become infected).
The nature of feed manufacturing has evolved from locally sourced to globally sourced feed ingredients; and some essential ingredients may originate from regions where disease outbreaks are active. With an increasing awareness of emerging animal diseases and their ability to cross national boundaries, there is a greater need for vigilance and improved mitigation methods.
What specific steps can be taken to mitigate risk? Of course, responsible sourcing is critical — feed and feed ingredients should be sourced from growers and suppliers that have proven, demonstrable quality and safety standards. Although some advocate that holding times can be effective, there is variability — and therefore, inherent risk — associated with this practice. Proposed holding times range from 15 days to nearly 400 days, depending on the ingredients in question, temperature, storage conditions and more. For many manufacturers and producers, these extended holding times simply may not be completely feasible.
Chemical mitigants have also been evaluated by research groups for the ability to inactivate viruses — formaldehyde and a medium-chain fatty acid (MCFA) additive among them (Sal CURB and CaptiSURE, Kemin Industries, Des Moines, Iowa). Research has demonstrated these chemicals can be effective at mitigating the risk of feed transmission of PEDV, porcine reproductive and respiratory syndrome virus and senecavirus A.
The portfolio of work on this subject is continuously expanding, and the resulting learnings will be key to providing the feed and animal production industries with viable risk mitigation options.
With many producers evaluating the risk of ingredients becoming contaminated, there is considerable and increasing interest in using these chemical mitigants.
If a decision is made to use a mitigant with nutritionally important ingredients, such as vitamins, it is of upmost importance to assure that it will not have a negative effect on the nutritional value of the ingredient.
Vitamins are essential micronutrients but are generally among the most labile feed supplements. Exposure to heat, humidity, light and elements during various manufacturing processes and storage can destroy sensitive vitamins.
Therefore, specific product forms have been developed to optimize fortification with the most labile vitamins, with appropriate considerations for chemical stabilization (e.g., esterification of vitamins A and E, incorporation of antioxidants) and/or physical protection (e.g., vitamin A cross-linked beadlet). Appropriate vitamin product form technologies are developed to achieve an optimum, delicate balance of improved stability without compromised nutrient bioavailability.
The story is no different when we look to improve the safety of these vitamins through use of mitigants — understanding the effects of mitigant application on the vitamins is critical to ensure that animals receive the desired amounts of these essential micronutrients.
To build on recent research in the space, DSM and Kemin partnered to investigate the impact of a MCFA product (CaptiSURE) application on high-quality vitamin and trace mineral (VTM) swine premixes ("Effect of CaptiSURE on Vitamin Stability and Enzyme Activity, PTP-179"). CaptiSURE is a new product used to provide an added energy source in livestock and poultry diets — it contains palm kernel oil and is comprised of a blend of MCFAs.
In this study, a 4 lb. per ton swine VTM premix containing a source of phytase (Ronozyme HiPhos GT, DSM Nutritional Products) was divided so that one half was treated with 2% MCFA product and one half was treated with 2% mineral oil (control). Then, one half of each treated premix was stored for 70 days under one of two storage conditions: ambient November through January temperatures (13-46°F) and humidity (> 60% relative humidity) or controlled conditions with high temperature (> 85°F) and low humidity (< 40% relative humidity).
Representative samples of each premix were collected and submitted for analysis of the vitamins and phytase at days 0, 30 and 70. After 70 days of storage, all the vitamins and phytase were retained at 77% or greater of their initial levels; and there were no differences between the control-treated and MCFA-treated premixes at any timepoint or across storage conditions.
These results indicate that the MCFA product had no detrimental effect on vitamin and phytase stability and — as part of a comprehensive biosecurity program — can be added to premixes as a replacement for mineral oil without compromising the nutritional value of premix ingredients.
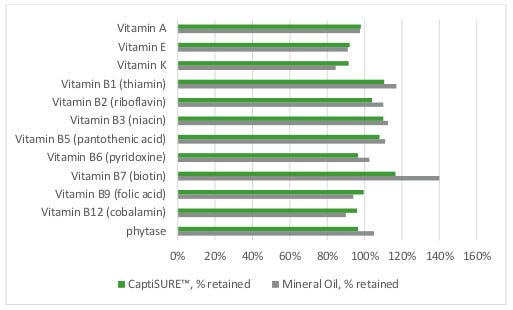
Figure 1. Effect of MCFA product or mineral oil on retention of vitamins, day 70 as percent retained from day 0.
The two different storage conditions used in this study were chosen to represent some of the existing possibilities for sheltered storage of premixes. Continuous storage under controlled, high temperature/low humidity conditions is likely to be considered for mitigating the risk of product contamination with pathogens. Alternatively, covered storage of premixes under midwestern, ambient winter conditions (cool temperatures and high humidity) is also possible.
In this study, under either of the two storage conditions tested, the stability of the vitamin and phytase product forms used were very good for the entire 70-day period. Previous research indicates that the extended storage of a VTM in hot and humid (ambient summer) conditions can result in significant reductions in the stability of vitamins and phytase, especially if sensitive vitamins and enzymes are provided as inferior product forms.
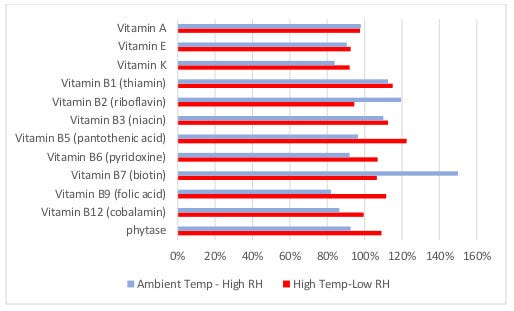
Figure 2. Effect of storage conditions on retention of vitamins, day 70 as percent retained from day 0.
These results are positive and impactful — building on existing research and confirming that treatment with a mitigant may be a viable option for producers aiming to reduce risk and to improve the safety of their feed ingredients and animals.
For questions or comments, please reach out to: Dr. Mark Bienhoff, Kemin Industries, [email protected] or Dr. Jon Bergstrom, DSM Nutritional Products, [email protected].
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
