thumbnail.png?width=700&auto=webp&quality=80&disable=upscale)
Agribusiness News.png?width=700&auto=webp&quality=80&disable=upscale)
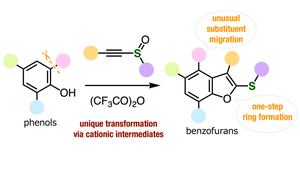
Natural Biologics expands to Africa and Middle EastNatural Biologics expands to Africa and Middle East
Natural Biologics expands into Africa and the Middle East with the addition of Mr. Zied Maalaoui to the sales team.
Subscribe to Our Newsletters
Feedstuffs is the news source for animal agriculture


.png?width=700&auto=webp&quality=80&disable=upscale)



.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)









.png?width=300&auto=webp&quality=80&disable=upscale)









.png?width=300&auto=webp&quality=80&disable=upscale)