thumbnail
Agribusiness News
Connect to build trust around the tough issuesConnect to build trust around the tough issues
Brian Fretwell, founder of Finding Good, shares a science-based approach of leading and connecting through smarter questions.
Subscribe to Our Newsletters
Feedstuffs is the news source for animal agriculture







.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)






.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)

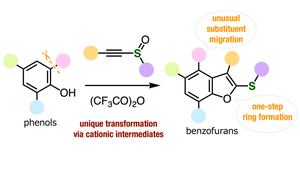

.png?width=300&auto=webp&quality=80&disable=upscale)




.png?width=300&auto=webp&quality=80&disable=upscale)
