By copying bacterial enzymes, a lab quickly learns to synthesize new class of antibiotic molecules the way nature does it.
June 2, 2017

The active component of penicillin and related antibiotics such as the cephalosporins — beta-lactams — is an "enchanted ring" called the beta-lactam ring. Antibiotics that include these rings are arguably the most important drugs in human history, having single-handedly increased global life expectancy by an estimated five years.
"People often say we're running out of antibiotics, but there are more than 20,000 molecules with antibiotic activity in the Handbook of Antibiotics," said Timothy Wencewicz, a chemist at Washington University in St. Louis (Mo.) who specializes in antibiotic design. "Fewer than 1% of those has ever been considered as a potential clinical candidate. They languish because it takes so much time and care to prepare a molecule for use as a pharmaceutical."
Wencewicz carefully chose one of these molecules, obafluorin, for further study. Oblafluorin, a beta-lactone discovered in 1984 by the Squibb Institute, is made by a fluorescent strain of soil bacteria that forms biofilms on plant roots.
Like penicillin, obafluorin has a four-membered ring, which puts strain on the bond angles that carbon prefers to adopt, Wencewicz explained.
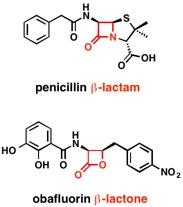
The beta-lactam ring couples three carbons and a nitrogen, whereas the beta-lactone ring consists of three carbons and an oxygen.
"The strain turns these rings into molecular bombs that go off when they are put in the right place at the right time, which is useful for killing microbes," he said.
However, because a four-member ring is unstable, these molecules are also short lived and hard to make. It took years for chemists to learn how to synthesize penicillin from chemicals and then to figure out how fungi make it. The antibiotic is still made by fermenting a penicillin-exuding strain of fungus in giant, stainless steel vats.
Wencewicz's laboratory was able to leapfrog the whole process by using genetics to zero in on the biosynthetic machinery bacteria use to make obafluorin and then to reconstruct that multi-step, enzyme-catalyzed process in the lab.
Wencewicz, graduate students Mars Reck and Jason Schaffer and undergraduate Neha Prasad described the complete biosynthetic machinery for the assembly of the beta-lactone obafluorin in the May 15 issue of Nature Chemical Biology.
The beta-lactones inhibit a large class of enzymes called the serine hydrolases. "There are hundreds of known serine hydrolases, and they are implicated in many human diseases," Wencewicz said. The beta-lactones may prove useful in the treatment of cancer and obesity as well as infectious diseases.
"We now have a complete enzymatic platform for making beta-lactone peptides from simple starting materials," Wencewicz said. "Since we know the gene sequences that code for this assembly line, we are using the power of modern genome sequencing to search for and make new beta-lactones made by other organisms."
Anyone familiar with the long and frustrating battle to produce enough penicillin to help wounded soldiers during World War II may marvel at the advances in genetics and chemistry that have allowed Wencewicz's lab to collapse the work of many decades into a few years.
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
